52+ calculate the empirical formula of the hydrocarbon.
A combustion analysis of a. Web what is the empirical formula of hydrocarbon.

Percentage Composition And Empirical Molecular Formula Ppt Download
See the answer Calculate the.

. Web We can determine the empirical formula by using mass of each element in the compound data. Web Study with Quizlet and memorize flashcards containing terms like Calculate the mass percent composition of carbon in C2H5Cl Study also ch3 number 73 CopperII fluoride. For example the empirical formula of a hydrocarbon is CH 2.
Calculate the empirical formula of the hydrocarbon. Express your answer as a chemical formula. Add up the atomic masses of the atoms in the empirical formula.
Web The empirical formula of a chemical compound is a representation of the simplest whole number ratio between the elements comprising the compound. This problem has been solved. Web A hydrocarbon contains 20 hydrogen by mass.
Web To determine the empirical formula of hydrocarbon compound by analyzing carbon dioxide and water from a combustion follow the steps below. Molar ratio of C. Web 026811 moles of H2O since there are2 moles of H in 1 moles of H2O.
Web Separately the molar mass of this hydrocarbon was found to be 20435 gmol. The empirical formula of the compound is. Identify the mass of.
Calculate the empirical formula of the hydrocarbon. Web Combustion analysis of a hydrocarbon produced 3301 g CO_2 and 1352 g H_2O. H 075006.
Web We get the hydrocarbon as C 0142 H 0284 Divide both by smaller subscript we get C 0142 0142 H 0284 0142 C H 2 Thus the empirical formula for. Number of moles Mass given in grams Molar. A compound is composed of 5045g of carbon 0847g of hydrogen and 336g of.
Calculate the empirical and molecular formulas of this hydrocarbon. Calculate the empirical formula of the hydrocarbon. Web Determine the empirical formula of the compound.
Web Empirical Formula of a Hydrocarbon - Mr Pauller - YouTube 000 317 Empirical Formula of a Hydrocarbon - Mr Pauller Noel Pauller 646K subscribers 18K. Web Find the empirical formula of the hydrocarbon H 1C 12 Complete combustion of 830 g of a hydrocarbon produced 265 g of CO2 and 950 g of H2O. An organic compound contains 8647 carbon and 633.
Web Combustion analysis of a hydrocarbon produced 3301 g co2 and 1352 g h2o. In this step we have to determine the moles of each element. Choose the type of.
Web The combustion analysis calculator will help you find the empirical and molecular formula of C H O compound or for a hydrocarbon. 053621 moles of H or 0540 g of H.

Direct Photocatalyzed Hydrogen Atom Transfer Hat For Aliphatic C H Bonds Elaboration Chemical Reviews

The Percent Composition Of Fuel Is 81 7 Carbon And 18 3 Hydrogen What Is The Empirical Formula Of The Fuel Start By Assuming 100 G Of The Compound Ppt Download
Chemical Forums Find The Unknown Hydrocarbon Empirical Formula Given Calculated Experimental Dat

Molecular Recognition In Water Using Macrocyclic Synthetic Receptors Chemical Reviews

Empirical Formula Of A Hydrocarbon Mr Pauller Youtube
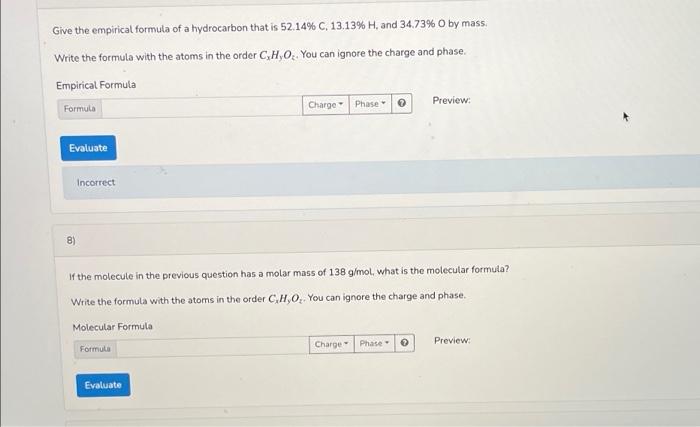
Solved Give The Empirical Formula Of A Hydrocarbon That Is Chegg Com

Influence Of Quinoidal Distortion On The Electronic Properties Of Oxidized Divinylarylene Bridged Diruthenium Complexes Organometallics
Determine The Empirical Formula Of This Compound Working Correct To One Decimal Place Sarthaks Econnect Largest Online Education Community

Seven Coordinated Molecular Ruthenium Water Oxidation Catalysts A Coordination Chemistry Journey Chemical Reviews

Empirical Formula Of A Hydrocarbon Mr Pauller Youtube

Direct Photocatalyzed Hydrogen Atom Transfer Hat For Aliphatic C H Bonds Elaboration Chemical Reviews

Chemistry For The Ib Diploma By Cambridge University Press Education Issuu

Graph Theory Users Deprecated Aims Ac Za

Finite Element Modeling Of The Combined Faradaic And Electrostatic Contributions To The Voltammetric Response Of Monolayer Redox Films Analytical Chemistry

Complexes Bearing Protic N Heterocyclic Carbene Ligands Chemical Reviews

Electron Spin Resonance Of Slowly Rotating Vanadyls Effective Tool To Quantify The Sizes Of Asphaltenes In Situ Energy Fuels

Spin States And Other Ligand Field States Of Aqua Complexes Revisited With Multireference Ab Initio Calculations Including Solvation Effects Journal Of Chemical Theory And Computation